The human skin is the second largest organ of the human body. The stratum corneum (SC) is the outmost layer of the human skin and performs various protective and adaptive physiological functions such as protecting against physical, chemical and biological damages.*
The homeostasis of the lipid matrix of the stratum corneum is essential for the correct functioning of the SC. If the composition of this lipid matrix is changed or disturbed skin ailments such as atopic dermatitis or psoriasis can be the result. According to various studies direct replenishment of the SC lipids on damaged skin has positive effects on the recovery of its barrier properties.*
Cerosomes or stratum corneum liposomes are a relatively new class of liposomes which are being investigated for the application as skin barrier repairing agents in chronical skin diseases.*
In the article “Cerosomes as skin repairing agent: Mode of action studies with a model stratum corneum layer at liquid/air and liquid/solid interfaces” Fabio Strati, Tetiana Mukhina, Reinhard H. H. Neubert, Lukas Opalka, Gerd Hause, Christian E. H. Schmelzer, Matthias Menzel, Gerald Brezesinski describe how they prepared cerosomes, i.E. liposomes composed of SC lipids in order to investigate the mechanism of interaction with a 2D model of the SC lipid matrix.*
After a first step of characterizing the used SC model monolayer in detail they carried out the development of stable SC liposomes, the so-called cerosomes, in a second step.*
Once the cerosome formulations were developed and characterized, the interaction between these and monolayers of the SC lipid matrix model was investigated.*
The interaction was probed by means of adsorption isotherms after subphase injection, and after the transfer to a solid support by atomic force microscopy (AFM) measurements.*
The AFM experiments were performed to gain information about the structures of the formed assemblies. This technique allows to resolve the lateral organization and to visualize the presence of lipid domains and/or adsorbed vesicles be performing topographic surface measurements of the sample deposited onto a solid support with an Angstrom resolution in transversal direction.*
Topographical images were recorded in liquid state using NANOSENSORS uniqprobe qp-BioT AFM probes in a standard liquid cell containing the needed buffer.*
The results obtained with the application of AFM showed that the liposomes were able to both penetrate into empty spaces and lower domains present in the SC model monolayer and get adsorbed at the monolayer forming localized multilayers.*
The results presented in the article indicate that a strong interaction occurred between SC monolayers and the cerosomes.*
The study proves for the first time the mode of action by which cerosomes exploit their function as skin barrier repairing agents on the SC.*
The use of such formulations might not only be limited to restore the damaged skin but they could be also used to deliver active pharmaceutical ingredients encapsulated in the cerosomes. This might open new and interesting scenarios for treating skin conditions such as inflammations caused by atopic dermatitis and/or psoriasis.*
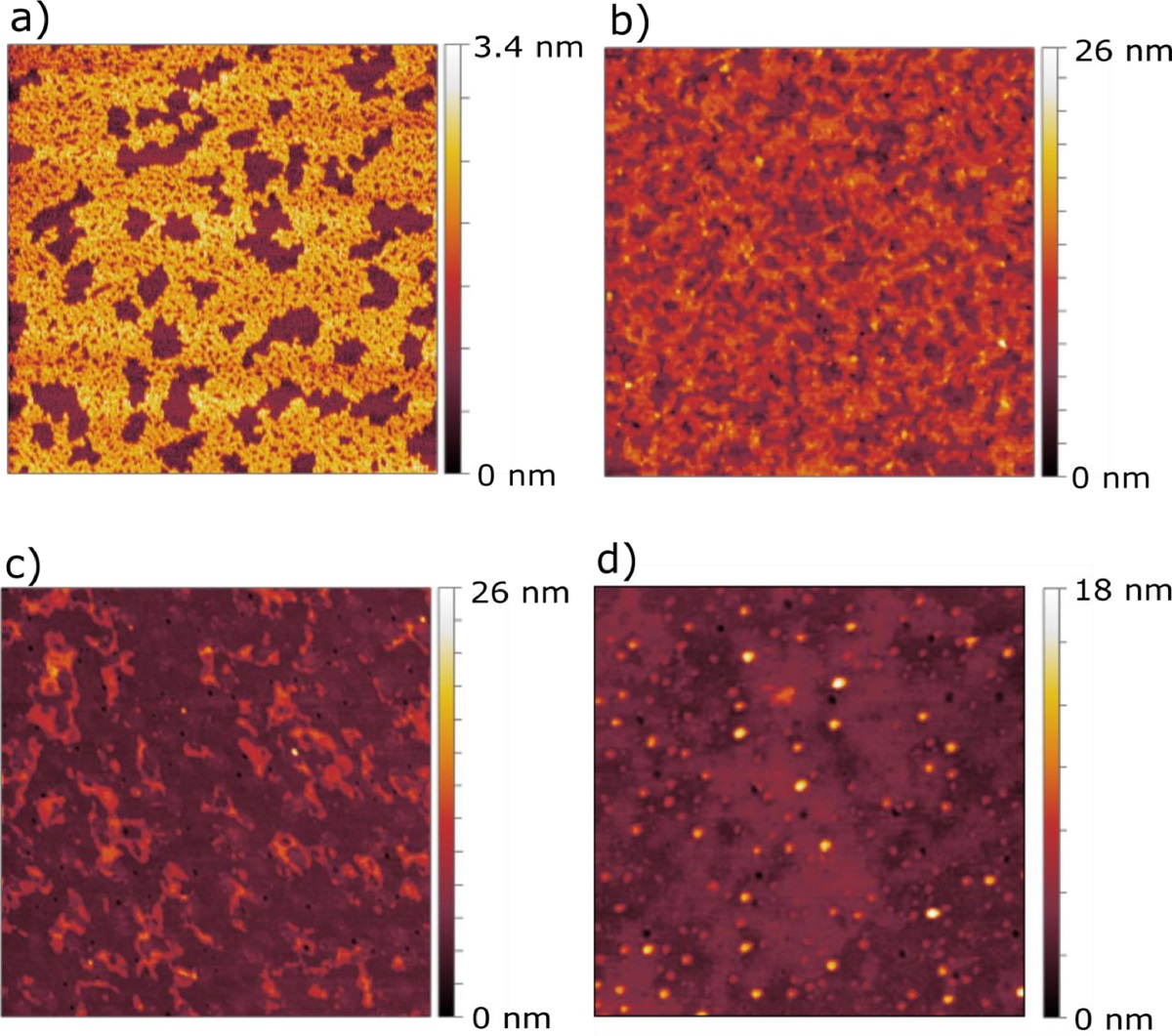
Fig. 7 from “Cerosomes as skin repairing agent: Mode of action studies with a model stratum corneum layer at liquid/air and liquid/solid interfaces” by F. Strati et al: AFM scans of a) SC model monolayer transferred via LB method onto mica support, b) SC model monolayer after injection of cerosomes, c) SC model monolayer after injection of cerosome + S75-3 formulation, and d) SC model monolayer after injection of S75-3 liposomal formulation. All samples were transferred via the LS method onto glass substrate. Each experiment was performed at 20°C and the subphase used for a) was Millipore water while for b), c), and d) the same aqueous solutions have been used as for the formulation of the liposomes.
*Fabio Strati, Tetiana Mukhina, Reinhard H. H. Neubert, Lukas Opalka, Gerd Hause, Christian E. H. Schmelzer, Matthias Menzel, Gerald Brezesinski
Cerosomes as skin repairing agent: Mode of action studies with a model stratum corneum layer at liquid/air and liquid/solid interfaces
BBA Advances, Volume 2, 2022, 100039
DOI: https://doi.org/10.1016/j.bbadva.2021.100039
Please follow this external link to read the full article: https://doi.org/10.1016/j.bbadva.2021.100039
Open Access: The article “Cerosomes as skin repairing agent: Mode of action studies with a model stratum corneum layer at liquid/air and liquid/solid interfaces” by Fabio Strati, Tetiana Mukhina, Reinhard H. H. Neubert, Lukas Opalka, Gerd Hause, Christian E. H. Schmelzer, Matthias Menzel, Gerald Brezesinski is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.