Mechanical forces are key regulators of cellular behavior and function, affecting many fundamental biological processes such as cell migration, embryogenesis, immunological responses, and pathological states. *
Specialized force sensors and imaging techniques have been developed to quantify these otherwise invisible forces in single cells and in vivo. However, current techniques rely heavily on high-resolution microscopy and do not allow interrogation of optically dense tissue, reducing their application to 2D cell cultures and highly transparent biological tissue. *
In the article “Deformable microlaser force sensing” Eleni Dalaka, Joseph S. Hill, Jonathan H. H. Booth, Anna Popczyk, Stefan R. Pulver, Malte C. Gather and Marcel Schubert introduce DEFORM, deformable microlaser force sensing, a spectroscopic technique that detects sub-nanonewton forces with unprecedented spatio-temporal resolution. *
DEFORM is based on the spectral analysis of laser emission from dye-doped oil microdroplets and uses the force-induced lifting of laser mode degeneracy in these droplets to detect nanometer deformations. *
The authors use gold-standard atomic force microscopy (AFM) measurements to validate the absolute scale of the extracted forces and to evaluate the optical response of individual droplets in order to be able to demonstrate the ability of DEFORM to extract absolute mechanical forces in a later step. *
To characterize the mechanical properties of the microlaser droplets, individual droplets were deformed under a well-defined force applied by an atomic force microscope (AFM, Fig. 2a cited in this blogpost) while simultaneously detecting the microlaser emission. *
From the AFM force-distance curves Eleni Dalaka et al. extracted the surface tension of individual droplets, finding a mean of 4.5 mN/m for droplets produced with 0.6 mM of Tween 20 surfactant (Fig. 2c, d of the cited article), in good agreement with pendant droplet tensiometer measurements (Supplementary Fig. S2 of the cited article). *
The authors then systematically increased the force applied by the AFM (Fig. 2e) and observed a more pronounced mode splitting that scaled linearly with the applied force (Fig. 2f), consistent with the analytical calculations (Fig. 1b of the cited article). *
The controlled deformations of single droplet microlasers were performed using an atomic force microscope which was installed on the inverted microscope that was used for the lasing experiments, allowing simultaneous optical and mechanical characterization of the droplets. *
For indentation, a 17 µm glass sphere was glued to the AFM tip of a soft AFM cantilever with nominal stiffness of k = 0.01 N/m (NANOSENSORS™ uniqprobe qp-SCONT). *
NANOSENSORS™ uniqprobe AFM probes offer an outstanding uniformity of the mechanical AFM cantilever characteristics which is particularly important for applications where a large number of AFM probes with known and near identical force constants or resonance frequencies are needed. The AFM cantilevers of the uniqprobe series are especially adapted for applications in molecular biology, biophysics and quantitative nano-mechanical studies.
The AFM cantilevers were calibrated prior to the bead attachment using the thermal method.
To stabilize the droplet microlasers on the glass substrate hydrogen bonds between the hydroxyl groups of Tween-20 on the droplet surface and the glass substrate were induced by lowering the pH of the PBS solution from 7 to 648. *
In a typical experiment, the AFM cantilever was approaching the droplet with a speed of 1 µm/s until a set force value was reached. This set value was then kept constant for 10 s during which 20 lasing spectra were acquired which allows to extract averaged lasing characteristics under the applied force. Lasing spectra before and after droplet compression were also recorded. To test the appearance of viscoelastic effects on the droplet lasers, we performed experiments with mechanical creep (200 pN applied load for either 10 s or 5 min) and different approach velocities (1, 10 and 100 µm/s). No significant viscoelastic effects were observed in these experiments. *
Measurements of the surface tension of microlasers were performed with a maximum force of 1 nN. The acquired force-distance curves were corrected for any linear shifts, and the contact point was set as the origin of the x-axis (distance). The data is then linearly fitted to extract the surface tension which is equal to the slope of the curve. *
Eleni Dalaka et al. also developed a model that links changes in laser spectrum to applied force and allows to extract the eccentricity of the flexible microlaser droplets. Based on the known deformation of the microlasers, the applied force can then be directly calculated. *
DEFORM is then used to measure forces in 3D and at depths of hundreds of microns within tumor spheroids and late-stage Drosophila larva.
Eleni Dalaka et al. furthermore show continuous force sensing with single-cell spatial and millisecond temporal resolution, thus paving the way for non-invasive studies of biomechanical forces in advanced stages of embryogenesis, tissue remodeling, and tumor invasion. *
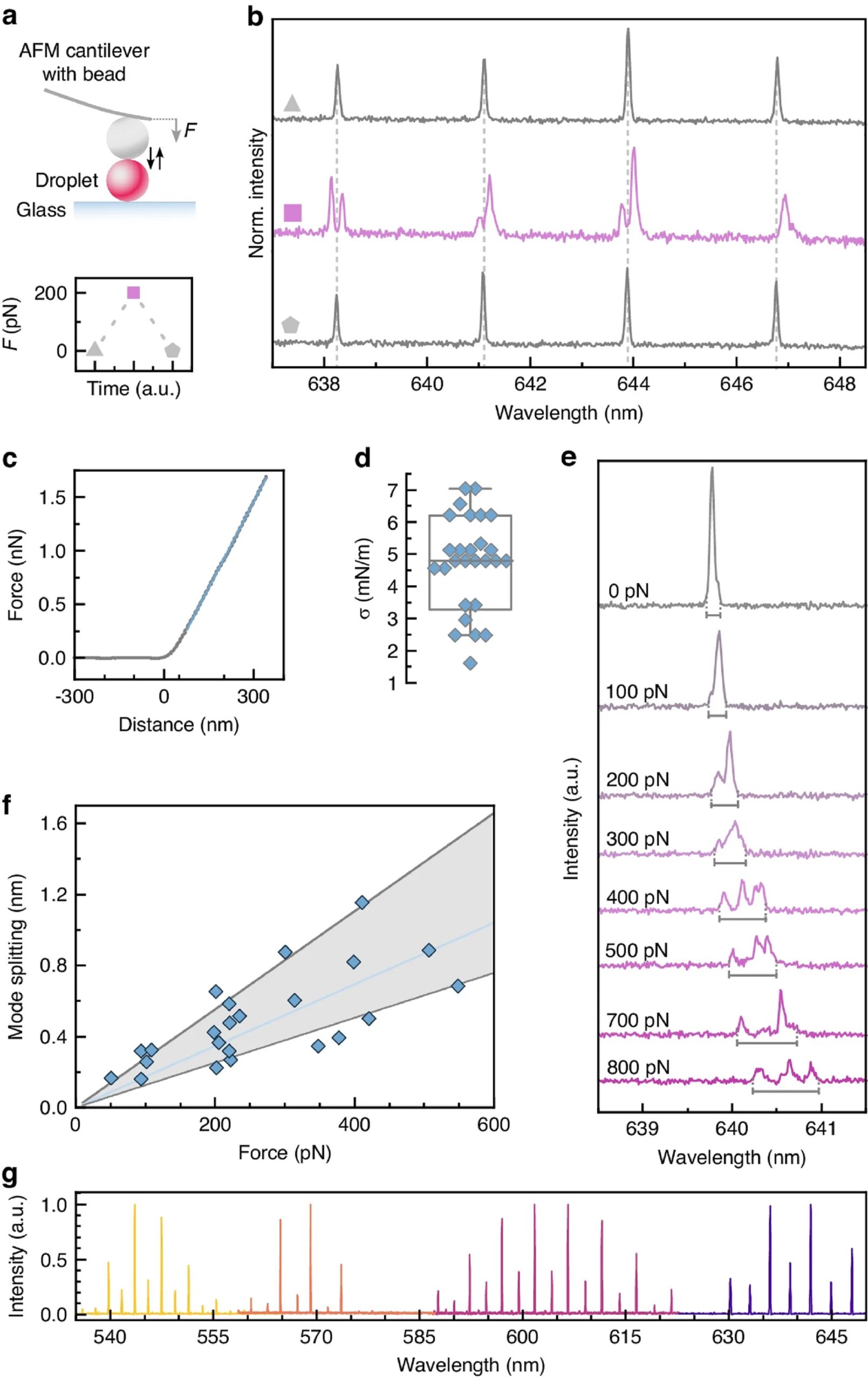
Fig. 2 from Eleni Dalaka et al. 2024 “Deformable microlaser force sensing”:
DEFORM reliably measures sub-nanonewton forces.
a Schematic illustration of the deformation of single microlaser droplets by an atomic force microscope (top) and visualization of a typical push-and-release experiment (bottom). b Microlaser spectra detected before, during, and after the application of a 200 pN force with the AFM (symbols as in a). c Typical force-distance curve used to calculate the stiffness of the droplets. d Variation in stiffness for a batch of droplet microlasers (N = 27). Boxplot showing the median and standard deviation, while whiskers represent the 5th and 95th percentile. e Evolution of the microlaser spectrum under increasing applied force. The gray bars below each spectrum indicate the fitted mode splitting, i.e. separation in wavelength between the leading and trailing edge of the mode. All spectra are plotted on the same scale but vertically offset for clarity. f Mode splitting versus force applied by AFM. A linear regression analysis is used to obtain the correlation between laser mode splitting and external force (solid blue line). The gray area marks the error of the measurement used for further calculations of the force. g Lasing spectra of undeformed microlasers doped with 4 different fluorescent dyes, C545T, BODIPY, Nile Red, Rhodamine B (left to right)
*Eleni Dalaka, Joseph S. Hill, Jonathan H. H. Booth, Anna Popczyk, Stefan R. Pulver, Malte C. Gather and Marcel Schubert
Deformable microlaser force sensing
Light: Science & Applications (2024) 13:129
DOI: https://doi.org/10.1038/s41377-024-01471-9
The article “Deformable microlaser force sensing” by Eleni Dalaka, Joseph S. Hill, Jonathan H. H. Booth, Anna Popczyk, Stefan R. Pulver, Malte C. Gather and Marcel Schubert is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.