In many tissues, cell shape and orientation are controlled by a combination of internal and external biophysical cues. *
Cell shape is closely controlled in different cell types and tissues and can impact cell phenotype, function, and fate. *
Anisotropic substrate topography is an ubiquitous cue that leads to cellular elongation and alignment, a process termed contact guidance whose underlying mechanisms remain incompletely understood. *
In medium and large arteries, atherosclerotic lesions initiate preferentially near branches and bifurcations where the endothelial cells (ECs) lining the blood vessels are cuboidal and randomly oriented. *
In contrast, zones where endothelial cells ECs are elongated and aligned in the direction of blood flow tend to be protected from the disease. *
These observations suggest a prominent shape-function relationship and motivate interest in elucidating the mechanisms governing the regulation of EC morphology and alignment. *
In their article “Distinct Contact Guidance Mechanisms in Single Endothelial Cells and in Monolayers” Claire Leclech, Apoorvaa Krishnamurthy, Laurent Muller, and Abdul I. Barakat seek to fill some of the remaining gaps in the understanding of endothelial cells shape regulation by microgrooves.
The authors try to find out, whether contact guidance responses are similar in single cells and in cellular monolayers.
To this end Leclech et al. used microgrooved substrates to investigate the contact guidance response of vascular endothelial cells (ECs) at densities ranging from single cells at an early stage of culture as well at a later stage when the cells attain confluence, form monolayers, and exhibit collective behavior. *
The fact that the authors follow the contact guidance response of ECs in time, from single cells to the formation and maturation of monolayers is the main novelty of their article. *
Claire Leclech et al. characterized the changes in intracellular organization associated with this transition and observed a disassembly of central focal adhesion (FAs) accompanied by a relocalization of aligned actin stress fibers (SFs) to the cell periphery.
These changes may be driven by the formation of cell–cell junctions, which are known to be physically associated with the actin cytoskeleton and with focal adhesion( FA)-associated proteins, but they may also be affected by the change in substrate stiffness associated with the secreted basement membrane (BM ) as evidenced by the Atomic Force Microscopy (AFM) experiments executed by the authors. *
By tracking EC shape on microgrooves in time and with increasing cell density, Claire Leclech et al. found that cell alignment and elongation progressively decrease, which they interpret as a gradual loss of response to substrate topography.*
The authors could show that contrary to common belief, focal adhesion (FAs) are not the principal elements involved in the prominent groove depth-dependent contact guidance in single ECs.*
Interestingly, the contact guidance response is greatly attenuated in confluent monolayers, and cell shape and alignment in that case are driven by the organization of the basement membrane (BM) secreted by the cells, which leads to a loss of cellular interaction with the microgrooves. The present finding of distinct contact guidance mechanisms in single ECs and in EC monolayers promises to inform strategies aimed at designing topographically patterned endovascular devices. *
For the characterization of the secreted basment membrane the surface topography and stiffness of the different samples were determined by Atomic force microscopy (AFM) with a commercially available scanning force microscope mounted on a fluorescent microscope. *
Atomic Force Microscopy measurements were performed using an AFM probe with circular symmetric rounded AFM tip with typical radius of curvature around 30 nm (0.03–0.09 N m−1) (uniqprobe qp-BioAC-CI, from NANOSENSORS). The spring constant was determined upon calibration by the thermal noise method. Quantitative imaging was conducted in water at 37 °C, with a setpoint of 0.3 nN for 60–80 ms. Young’s modulus values were extracted using the Hertz model (Poisson’s ratio of 0.5) and averaged over regions of approximately 20 × 20 µm.*
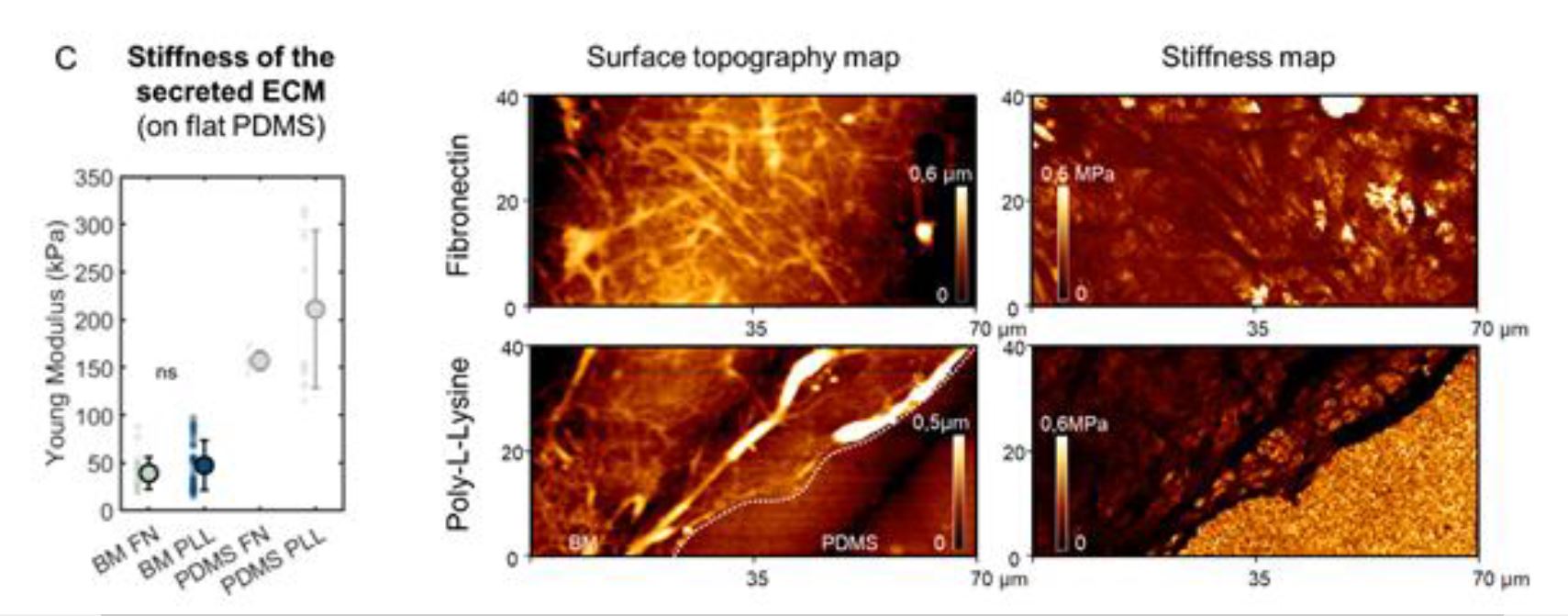
Supplementary Figure 10 from Claire Leclech et al. (2023) “Distinct Contact Guidance Mechanisms in Single Endothelial Cells and in Monolayers”:
Characterization of the secreted basement membrane.
(C) Surface topography and stiffness (Young’s modulus) obtained by atomic force microscopy of the secreted ECM or bare PDMS after 72 h of culture on flat surfaces coated with fibronectin (FN) or poly-L-lysine (PLL). Dots represent individual measures on n=2 independent experiments. Error bars represent standard deviations.
Please follow the link to the full article to find the full supplementary figure 10 and its description.
*Claire Leclech, Apoorvaa Krishnamurthy, Laurent Muller, and Abdul I. Barakat
Distinct Contact Guidance Mechanisms in Single Endothelial Cells and in Monolayers
Advanced Materials Interfaces 2023, 10, 2202421
DOI: https://doi.org/10.1002/admi.202202421
Open Access: The article “Distinct Contact Guidance Mechanisms in Single Endothelial Cells and in Monolayers” by Claire Leclech, Apoorvaa Krishnamurthy, Laurent Muller, and Abdul I. Barakat is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.