The female reproductive tract (female-RT) must decipher the repertoire of molecular cues received from the male during copulation in order to activate and coordinate tract functionality necessary for high fertility. In Drosophila, this modulation is partially driven by spermathecal secretory cells (SSC). The SSC are a layer of cuboidal secretory glandular cells surrounding the spermatheca capsule where sperm is stored. It is unclear, however, how the SSC regulate the system’s activity. *
In the article “Male-female communication enhances release of extracellular vesicles leading to high fertility in Drosophila” Javier Arturo Sanchez-Lopez, Shai Twena, Ido Apel, Shani Chen Kornhaeuser, Michael Chasnitsky, Andras G. Miklosi, Perla J. Vega-Dominguez, Alex Shephard, Amir Hefetz and Yael Heifetz show that mating activates the secretory machinery of the SSC.*
The SSC release a heterogeneous population of extracellular vesicles (EVs) which is involved in initiating and managing the increase in egg-laying, and possibly sperm storage. Moreover, sperm and male accessory gland proteins are essential for such mating-mediated SSC activity. Thus, mating regulates secretory/endocytic pathways required for trafficking of vesicles to SSC-female-RT target sites, which modulate and coordinate reproductive tract activity to achieve high fertility. *
The authors used atomic force microscopy to scan the extracellular vesicles (EVs).
The samples were scanned in liquid using AFM cantilever beam 3 (CB3) of the NANOSENSORS uniqprobe qp-BioAC-CI AFM probes. The qp-BioAC-CI AFM tips have been rounded to a nominal AFM tip radius of 30nm and are dedicated for cell imaging applications and measurements on soft and life science samples. The uniqprobe qp-BioAC-CI AFM probes feature three different rectangular AFM cantilevers on one side of the support chip and the cantilevers unite fairly high resonance frequencies with low force constants.
The reflective gold coating deposited on the detector side of the AFM cantilevers covers only the free end above where the AFM tip is located. Main advantages of the uniqprobe coating are considerably less AFM cantilever bending and reduced thermal drift particularly for measurements in liquid environments.
The samples were also scanned in QI mode, in which a force curve is measured on every pixel.*
From these force curves, the authors calculated the adhesion force and Young’s modulus. *
The profiles of adhesion and Young’s modulus were created by measuring the mean value of the particle in three different areas of 5 × 5 µm. The diameter (nm) was obtained by creating a cross section of each EV and measuring the base of the profile. *
The involvement of EVs in SSC-female-RT routes of communication has added another layer of complexity to the process driving the switch towards a functional female-RT, and to high fertility. The precise mechanism by which male-derived signals, including EVs, affect SSC-derived trafficking has yet to be resolved. Deciphering male-female communication via EVs in Drosophila and other organisms will contribute greatly to our understanding of the different combinations of input modalities and output networks leading to high fertility. *
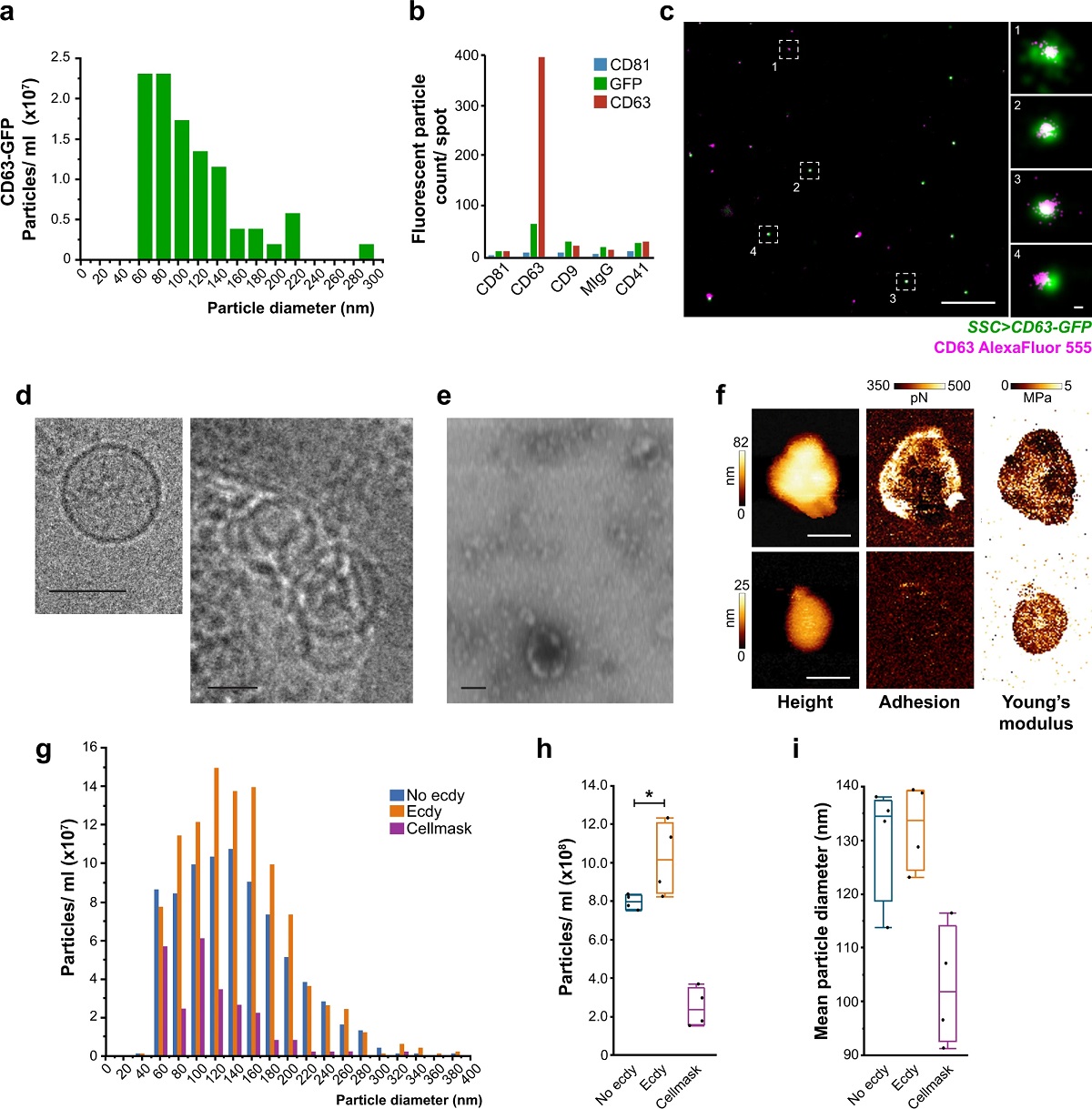
Fig. 5 from “Male-female communication enhances release of extracellular vesicles leading to high fertility in Drosophila” by Javier Arturo Sanchez-Lopez et al.:
The spermatheca releases EVs with specific characteristics.
a–e The spermathecae (SSC > CD63-GFP) were cultured ex vivo to characterize the particles that were released to the spent media: a size distribution of CD63-GFP-positive particles (n = 25 spermathecae from 15 flies) using single-particle tracking of the Nanoimager. b Characterization of CD63-GFP particles by ExoView (100 spermathecae from 50-60 flies) using anti-GFP or anti-CD63. c Representative dSTORM image of EVs found in the spent media of SSC expressing CD63-GFP (n = 25 spermathecae from 15 flies), stained with AlexaFluor555 conjugated anti-CD63 primary antibody. The image shows CD63-GFP-positive EVs and single CD63 AlexaFluor555 molecules the EV’s surface. Scale bars = 1 µm and insets = 20 nm. d–e The morphology of EVs in the spent media was observed by e negative staining in STEM; scale bar = 200 nm; and by d Cryo-TEM; Scale bar = 100 nm (see also Supplementary Fig. 5a-c). f–i The spent media of ex vivo cultured spermathecae were analyzed for the presence of EVs: f Representative AFM scans of SSC-EVs isolated by acoustic sorting (n = 100 spermathecae from 70 flies), showing from left to right: height (nm), adhesion (pN) and Young’s modulus images (MPa). Scale bars = 100 nm (see Supplementary Fig. 5d–g). g–i Single-particle tracking in the nanoimager. g CellMaskTM-positive particle size distribution and concentration profiles of EVs in the spent media of spermathecae incubated in media alone (No ecdy) or with ecdysone (Ecdy) (see also Supplementary Movie 5 for a time lapse of spermathecae end apparatus and endosomal activity post-ecdysone stimulation and methods, ex vivo spermatheca culture section); media with only CellMaskTM stain served as a control for the formation of dye aggregates. h Particle concentration and i mean particle diameter (nm) from g; Box plots are the measurements of particles from four frames of spent media from 25 spermathecae; boxes represent maximum, median and minimum values with outliers; one-way ANOVA, with nonparametric Wilcoxon multiple comparison post-hoc test; *p < 0.0001.
*Javier Arturo Sanchez-Lopez, Shai Twena, Ido Apel, Shani Chen Kornhaeuser, Michael Chasnitsky, Andras G. Miklosi, Perla J. Vega-Dominguez, Alex Shephard, Amir Hefetz and Yael Heifetz
Male-female communication enhances release of extracellular vesicles leading to high fertility in Drosophila
Nature Communications Biology volume 5, Article number: 815 (2022)
DOI: https://doi.org/10.1038/s42003-022-03770-6
Please follow this external link to read the full article: https://rdcu.be/cYtUq
Open Access: The article “Male-female communication enhances release of extracellular vesicles leading to high fertility in Drosophila” by Javier Arturo Sanchez-Lopez, Shai Twena, Ido Apel, Shani Chen Kornhaeuser, Michael Chasnitsky, Andras G. Miklosi, Perla J. Vega-Dominguez, Alex Shephard, Amir Hefetz and Yael Heifetz is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.