Morphogenesis requires embryonic cells to generate forces and perform mechanical work to shape their tissues. Incorrect functioning of these force fields can lead to congenital malformations.*
Understanding these dynamic processes requires the quantification and profiling of three-dimensional mechanics during evolving vertebrate morphogenesis.*
In the article “Quantifying mechanical forces during vertebrate morphogenesis” Eirini Maniou, Silvia Todros, Anna Urciuolo, Dale A. Moulding, Michael Magnussen, Ioakeim Ampartzidis, Luca Brandolino, Pietro Bellet, Monica Giomo, Piero G. Pavan, Gabriel L. Galea and Nicola Elvassore describe elastic spring-like force sensors with micrometre-level resolution, fabricated by intravital three-dimensional bioprinting directly in the closing neural tubes of growing chicken embryos.*
Integration of calibrated sensor read-outs with computational mechanical modelling allows direct quantification of the forces and work performed by the embryonic tissues. As they displace towards the embryonic midline, the two halves of the closing neural tube reach a compression of over a hundred nano-newtons during neural fold apposition. Pharmacological inhibition of Rho-associated kinase to decrease the pro-closure force shows the existence of active anti-closure forces, which progressively widen the neural tube and must be overcome to achieve neural tube closure. *
Overall, the author’s approach and findings highlight the intricate interplay between mechanical forces and tissue morphogenesis.*
The atomic force microscopy (AFM) described in the article was conducted using a commercially available atomic force microscope.*
The force-displacement curves were acquired using NANOSENSORS™ PointProbe® Plus PPP-CONTSCR silicon AFM probes with a typical spring constant of 0.2 N/m. *
The AFM cantilever spring constants were calibrated by the manufacturer prior to use. The sensitivity of each AFM cantilever was adjusted by measuring the slope of the force-distance curve acquired on a hard reference material prior to each experiment. *
Indentation experiments were repeated at least three times for each sample, at different locations. All AFM measurements were done in a fluid environment (PBS) at room temperature.*
The Young’s modulus was calculated by applying a fit of the Hertz model to the force–distance curve, assuming a Poisson ratio of 0.5, as is common practice for PEG hydrogels. Preliminary in silico analyses of the AFM testing procedure were carried out to evaluate the effects of boundary conditions on the estimation of Young’s modulus.*
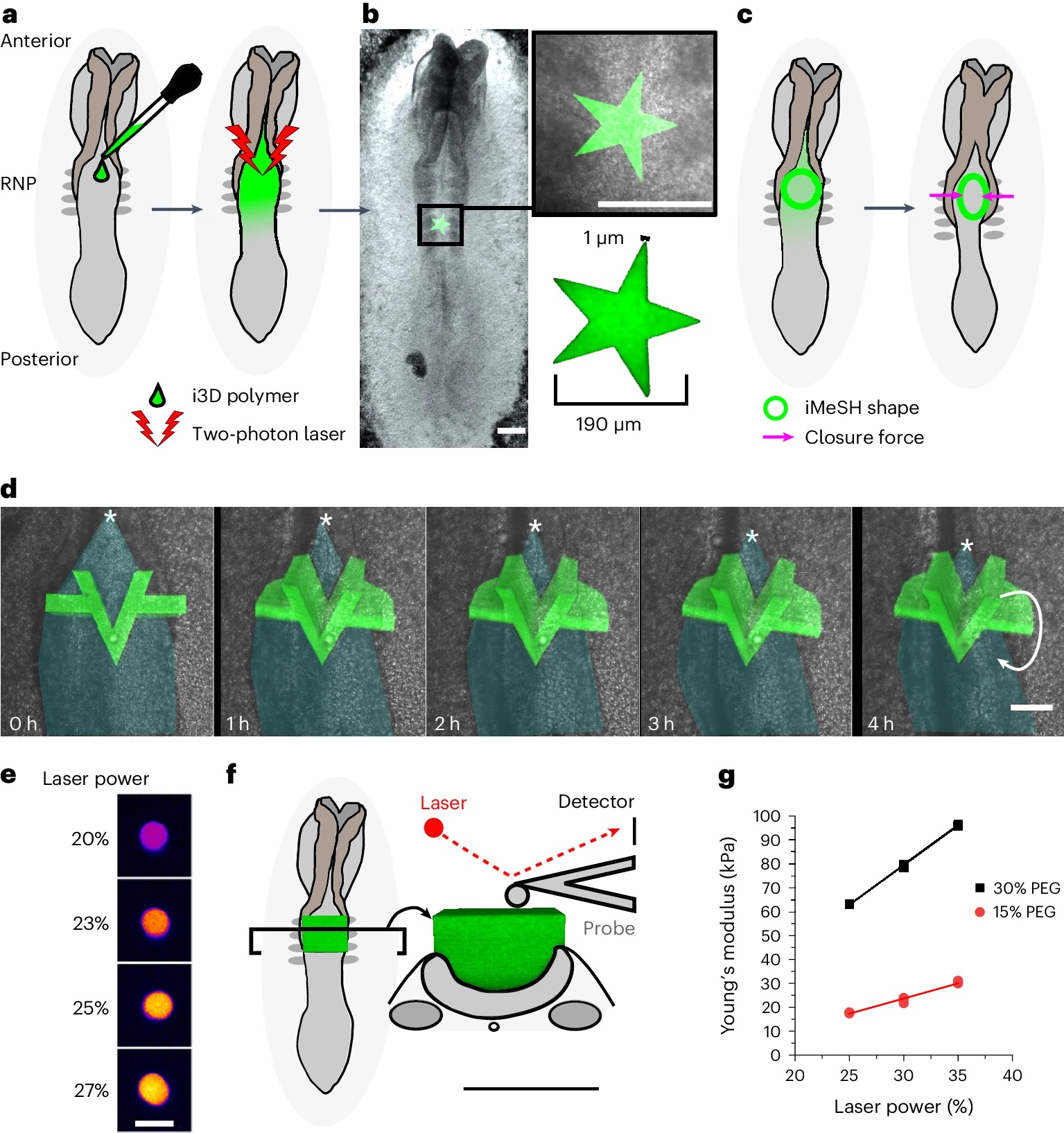
Fig. 1 from : Eirini Maniou et al. 2024 “Quantifying mechanical forces during vertebrate morphogenesis”:
The i3D bioprinting with accurately determined position, geometry and stiffness.
a, Schematic of a chicken embryo illustrating the experimental workflow: 2–3 µl i3D polymer is pipetted directly onto the rhombocervical neuropore (RNP) and photo-crosslinked with a two-photon laser. The iMeSH structures are shown in green throughout. b, Stereoscope image of an embryo with a star shape photo- crosslinked on the flat neural plate. Scale bars, 200 µm. The star dimensions are indicated in the inset. c, Schematic showing iMeSH compression by apposition of the neural folds. d, Time-lapse images showing the sequential displacement of a rigid iMeSH shape, shown as a 3D confocal reconstruction superimposed on the embryo imaged with transmitted light. Cyan shading, open neural tube; *, zippering point; arrow indicates rotation of the printed shape; scale bar, 50 µm. The times are shown. e, Fire lookup table showing the autofluorescence of iMeSH photo-crosslinked with the indicated laser powers on the same embryo. Scale bar, 25 µm. f, Schematic illustration of AFM stiffness testing of an iMeSH shape; 3D reconstructions of the shape are shown superimposed on a dorsal and transverse schematic of the embryo. Scale bar, 100 µm. g, AFM quantification of iMeSH crosslinked on an embryo at the indicated laser powers. The values were calculated from AFM indentations performed at a rate of 0.5 μm s–1 and depths of 1 μm (30% 7-hydroxycoumarin-3-carboxylic acid (HCC) polyethylene glycol (PEG)) or 2 μm (15% PEG).
*Eirini Maniou, Silvia Todros, Anna Urciuolo, Dale A. Moulding, Michael Magnussen, Ioakeim Ampartzidis, Luca Brandolino, Pietro Bellet, Monica Giomo, Piero G. Pavan, Gabriel L. Galea and Nicola Elvassore
Quantifying mechanical forces during vertebrate morphogenesis
Nature materials (2024)
DOI: https://doi.org/10.1038/s41563-024-01942-9
The article “Quantifying mechanical forces during vertebrate morphogenesis” by Eirini Maniou, Silvia Todros, Anna Urciuolo, Dale A. Moulding, Michael Magnussen, Ioakeim Ampartzidis, Luca Brandolino, Pietro Bellet, Monica Giomo, Piero G. Pavan, Gabriel L. Galea and Nicola Elvassore is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.