ZnFe2O4 is a well-investigated, versatile material with a spinel structure, which reportedly shows electrical conductivity in the range of 5 to 10 mS cm−1, a relatively small electronic bandgap of about 1.9 eV and values of 2.02 eV and 2.33 eV for the indirect and direct optical band gap. *
Apart from the low production price, the high and globally uniform availability of the constituents, high chemical stability against air or moisture and the harmlessness with regard to health and the environment, these characteristics make it an ideal candidate for photocatalytic or energy harvesting applications. *
In addition, nanostructured ZnFe2O4 has gained interest because depending on the size of the nanostructure and the synthesis method, Zn and Fe can partially exchange sites in the crystal structure. The degree of cation exchange allows tuning of the electronic and spin structure of the material making it interesting for various uses ranging from spintronic and microwave applications to sensor materials. *
In the field of energy storage, ZnFe2O4 has previously been discussed as possible material for the negative electrode in lithium-ion batteries (LIB). Even though its high theoretical capacity of 1072 A h kg−1 as well as its low toxicity compared to conventional Co- and Ni-containing compounds and the low production costs make it highly attractive, further investigations showed that the cycle stability is less than ideal, because the ZnFe2O4 breaks down to ZnO and Fe2O3 during repeated charging and discharging in a cell and metal ion dissolution takes place. *
In addition, the energy efficiency is very low. Still, various studies have been conducted that either condone decomposition since even the decay products still work well as negative electrode or do not charge and discharge over the full voltage window (shallow cycling) to prevent decomposition. *
In the article “Teaching an old dog new tricks: Ti-doped ZnFe2O4 as active material in zinc ion batteries – a proof of concept” Susanna Krämer, Julia Hopster, Anna Windmüller, R.-A. Eichel, M. Grünebaum, T. Jüstel, M. Winter and Kerstin Neuhaus investigate the suitability of the spinel material ZnFe2O4 for use as active material for the cathode side in zinc ion batteries. *
In addition to pure ZnFe2O4, part of the Fe3+ was doped with Ti4+ to achieve stabilization of Zn vacancies in the material and increase ionic conductivity as indicated by previous modelling results. *
For their study Susanna Krämer et al. produced samples with the compositions ZnFe2−xTixO4 (with x = 0 to 0.25) by a Pechini synthesis route. The crystal structure, microstructure, optical and electrochemical characteristics of the material were analyzed and compared to literature results. *
It has been successfully demonstrated that both pure and Ti doped ZnFe2O4 can be used as active material in the positive electrodes of zinc metal batteries or in an ‘‘anode-free’’ setup with Sn metal. *
Cells with calcined ZnFe2xTixO4 (x = 0.09)|0.5 M zinc triflate in acetonitrile|Zn showed a stable cycling behavior over 1000 cycles and an average initial specific capacity of 55 mA h g1.*
In their study the authors found pure zinc ferrite and material with low Ti concentrations (specifically x = 0.09) to work as active material in ZIBs. Samples above the solubility limit of Ti (in our case x = 0.13) did not show a stable cycling behavior. Addition of higher amounts of Ti was hence not deemed favorable to improve application of ZnFe2O4 as active material in zinc batteries. *
From studies on the use of ZnFe2O4 and ZnMn2O4 in various electrochemical applications, a variety of synthesis methods are already known for micro-structuring the material in order to increase the surface area and enable more effective use, ideally in combination with an electron conductive coating. This is a future target to considerably improve performance, especially the capacity, of ZnFe2O4-based materials for use in zinc metal and zinc ion batteries. *
Kelvin probe force microscopy (KPFM) measurements of ZFO, ZFTO7 and ZFTO13 showed a slight decrease in work function with increasing Ti content (Fig. 5a cited below). In addition, it was observed that for all measured samples, the grain boundaries showed a lower surface potential compared to the grain interiors (Fig. 5b). The difference was evaluated for 25 different grain boundary position in the three samples, but the potential difference for the samples did not depend on the Ti concentration and was roughly in the range of 25 mV, but with a large variation between individual grain boundaries (cf. Fig. S2, ESI†). It can therefore be assumed that this difference is merely due to band bending, which can typically be found at grain boundaries due to the different crystallographic orientation of adjacent grains. *
Kelvin Probe Force Microscopy (KPFM) measurements were performed in an Ar atmosphere using a commercially available atomic force microscopy with conductive NANOSENSORS PointProbe® Plus PPP-NCSTPt AFM tips. *
The samples were used as received without further surface modification and were dried in an Ar stream in the instrument before starting the measurements. KPFM measurements yield data about the local surface potential of a sample, which under ideal conditions is directly related to the Volta potential. If Ti4+ works as an n-type dopant in ZnFe2O414 an increasing work function (which means a decreasing surface potential) with increasing Ti4+ content can be expected.
Before and after the measurements, the AFM tip was calibrated on a freshly cleaved highly ordered pyrolytic graphite reference surface to minimize influence of AFM tip wear on the results. *
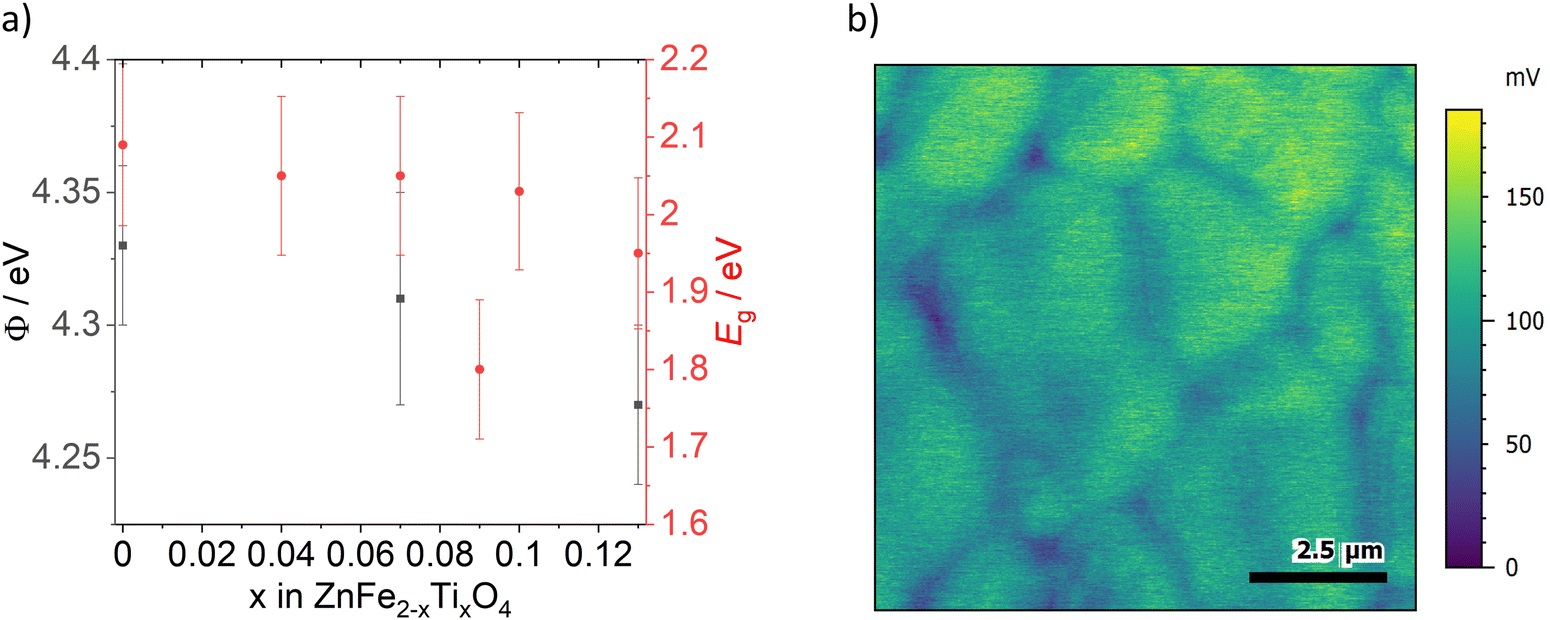
Fig. 5 from “Teaching an old dog new tricks: Ti-doped ZnFe2O4 as active material in zinc ion batteries – a proof of concept” by Susanna Krämer et al. 2024:
(a) Work function (Φ) measurements of ZFO, ZFTO7 and ZFTO13 (black squares) and data for the band gap energies (Eg) calculated from the Tauc plot for all samples except ZFTO25 (red circles). Error bars of work function are standard deviation from the average value. (b) Exemplary KPFM image of ZFO with grain boundaries showing lower surface potential (blue) than grain interior (green).
*Susanna Krämer, Julia Hopster, Anna Windmüller, R.-A. Eichel, M. Grünebaum, T. Jüstel, M. Winter and Kerstin Neuhaus
Teaching an old dog new tricks: Ti-doped ZnFe2O4 as active material in zinc ion batteries – a proof of concept
Energy Advances, 2024, Advance Article
DOI: https://doi.org/10.1039/D4YA00134F
The article “Teaching an old dog new tricks: Ti-doped ZnFe2O4 as active material in zinc ion batteries – a proof of concept” by Susanna Krämer, Julia Hopster, Anna Windmüller, R.-A. Eichel, M. Grünebaum, T. Jüstel, M. Winter and Kerstin Neuhaus is licensed under a Creative Commons Attribution 3.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/3.0/.