Ice plays a crucial role in our environment, with natural ice formations, such as glaciers, permafrost, river ice, and snow, strongly influencing the temperature, humidity, and weather patterns on Earth.*
Because of its importance in our lives, extensive experimental and theoretical studies have been conducted to understand the characteristic properties of ice.*
An in-depth analysis of the interface between ice and water is essential for a complete understanding of ice near-natural conditions.*
In the article “The interface between ice and alcohols analyzed by atomic force microscopy” Ryo Yanagisawa, Tadashi Ueda, Kei-ichi Nakamoto, Zhengxi Lu, Hiroshi Onishi and Taketoshi Minato investigate the interface between ice and organic solvents using atomic force microscopy (AFM).*
Atomically flat ice surfaces were prepared and observed by AFM in 1-octanol, 1-hexanol, and 1-butanol.
The results show differences in surface roughness influenced by the interaction of ice and alcohols.
The Young’s modulus of ice was analyzed by force curve measurements, providing valuable insights into the properties of ice in liquid environments.
To analyze the physical properties of surfaces and interfaces, atomic force microscopy (AFM) is emerging as a powerful tool due to its superior spatial resolution and sensitivity in detecting structural, mechanical, electronic, and magnetic properties.
Recent advances in AFM techniques for liquid environments make it a promising avenue for investigating the ice–water interface.
However, the analysis of the ice–water interface under realistic conditions by AFM is challenging, especially with respect to the fluctuation of the interface position when held at the freezing point.
To address this challenge, the authors of the article propose to use AFM on ice immersed in organic solvents. For example, when ice is in contact with 1-octanol (C8H18OH), it remains liquid below the freezing point of ice (273.15 K) and above the freezing point of 1-octanol (258 K).
The atomic force microscopy (AFM) measurements were conducted with a commercially available AFM that was placed in an acoustic enclosure and was cooled with the vapor of liquid nitrogen and a copper tube cooled with antifreezing fluid to maintain the environmental temperature at 264.7–270.2 K.
Topographic images were obtained in the amplitude modulation mode with NANOSENSORS PointProbe® Plus PPP-NCHAuD AFM probes with gold coating on the detector side of the AFM cantilever.
Throughout the process of acquiring the topographic image, the amplitude of the AFM cantilever’s oscillation was maintained at 17%–75% of its initial amplitude before the approach.
The spring constant of each lever was calibrated from the Brownian motion of the AFM cantilever. To analyze Young’s modulus from the force curve, the deflection sensitivity and AFM tip radius were calibrated using Young’s modulus of mica (70 GPa).
To confirm the reproducibility of the atomic force microscopy results, the measurements were performed on 6–32 points (the distance between the points was more than 100 µm) from 3–16 ices under each condition.
Although the interface between alcohols and ice is different from that between water and ice, Ryo Yanagisawa et al. expect that careful selection of suitable organic solvents will lead to new insights that mimic essential features of the ice–water interface.*
Ryo Yanagisawa et al. established an experimental system to analyze the interface between ice and alcohols (1-octanol, 1-hexanol, and 1-butanol) using atomic force microscopy.
They observed the surface structure of ice in liquid environments and demonstrated the analysis of physical properties, such as Young’s modulus, through force curve measurements in these liquid systems.
The results showed the characteristics of the ice surface in different solvents, suggesting potential applications in understanding surface and interface phenomena associated with ice under realistic conditions.*
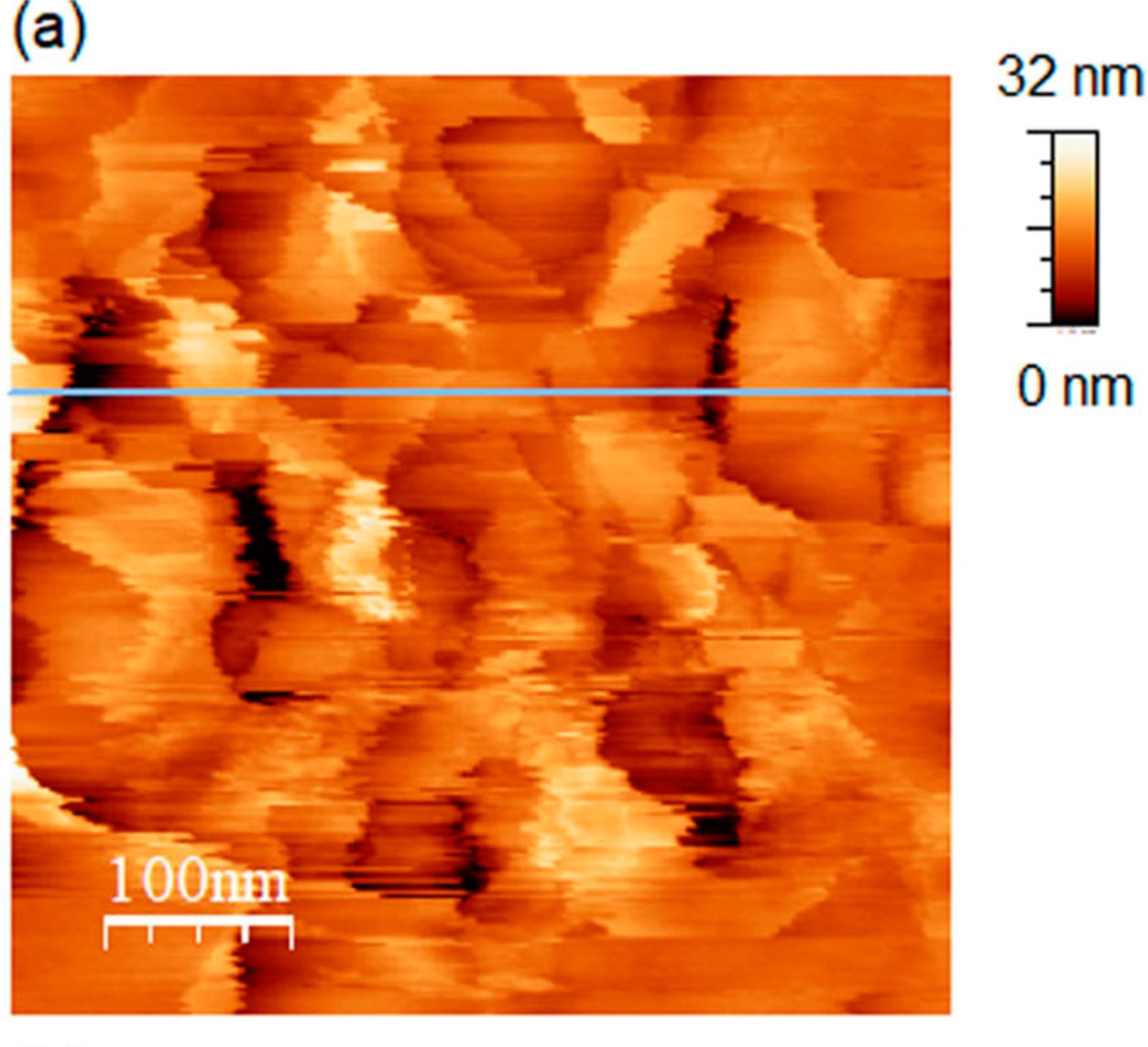
Figure 1 (a) from Ryo Yanagisawa et al. (2024) “The interface between ice and alcohols analyzed by atomic force microscopy”:
(a) AFM image (500 × 500 nm2) of the ice surface obtained in N2 at 267.1 K.
for 1 b) The line profile of panel (a). please refer to the cited article
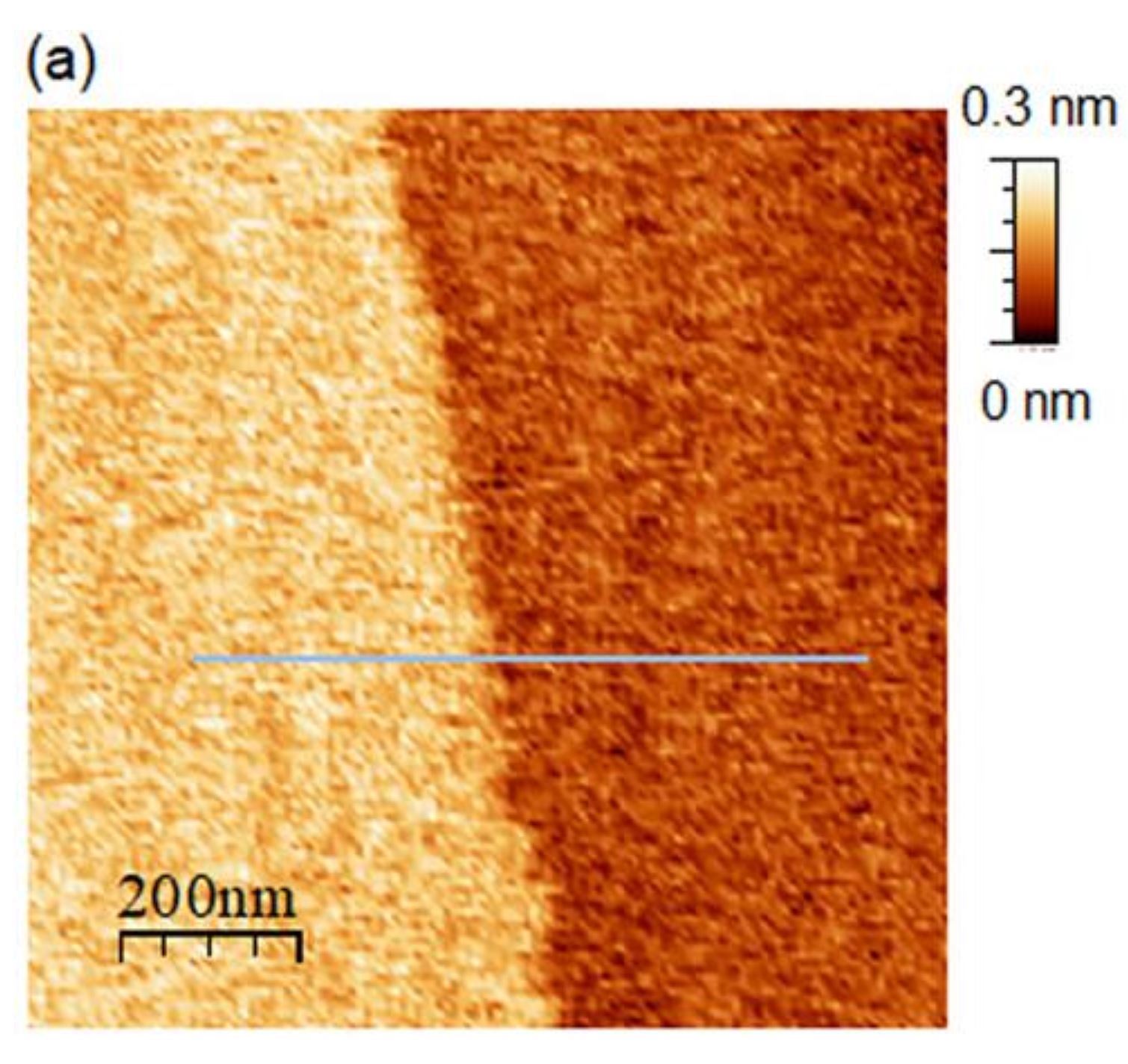
Figure 2 (a) from Ryo Yanagisawa et al. (2024) “The interface between ice and alcohols analyzed by atomic force microscopy”:
(a) AFM image (1 × 1 µm2) of the ice surface in 1-octanol at 265.9 K.
for (b) The line profile of panel (a) please refer to the cited article
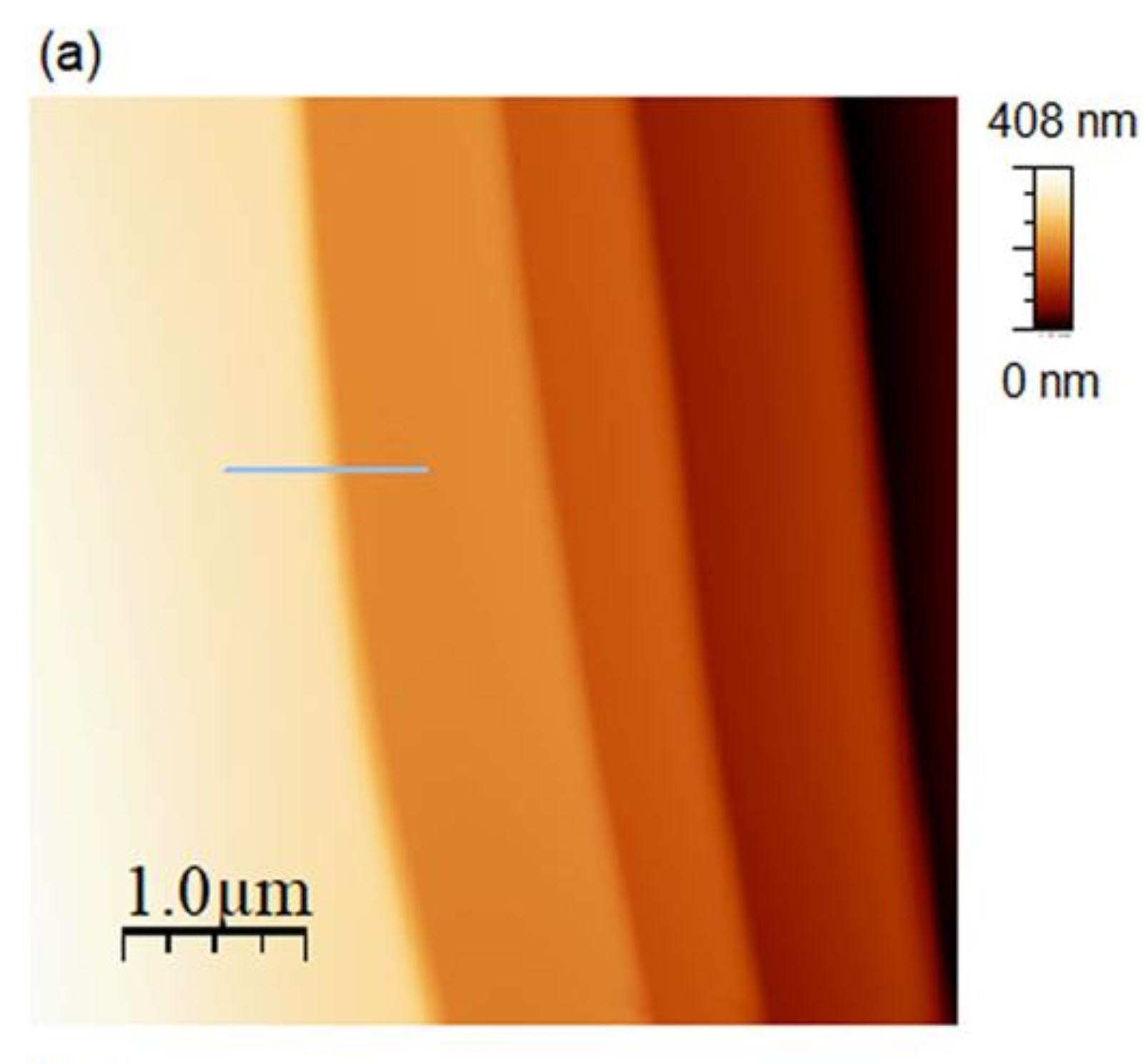
Figure 3 (a) from Ryo Yanagisawa et al. (2024) “The interface between ice and alcohols analyzed by atomic force microscopy”:
(a) AFM image (5 × 5 µm2) of the ice surface obtained ice in 1-hexanol at 270.2 K.
for (b) The line profile of panel (a) please refer to the cited article
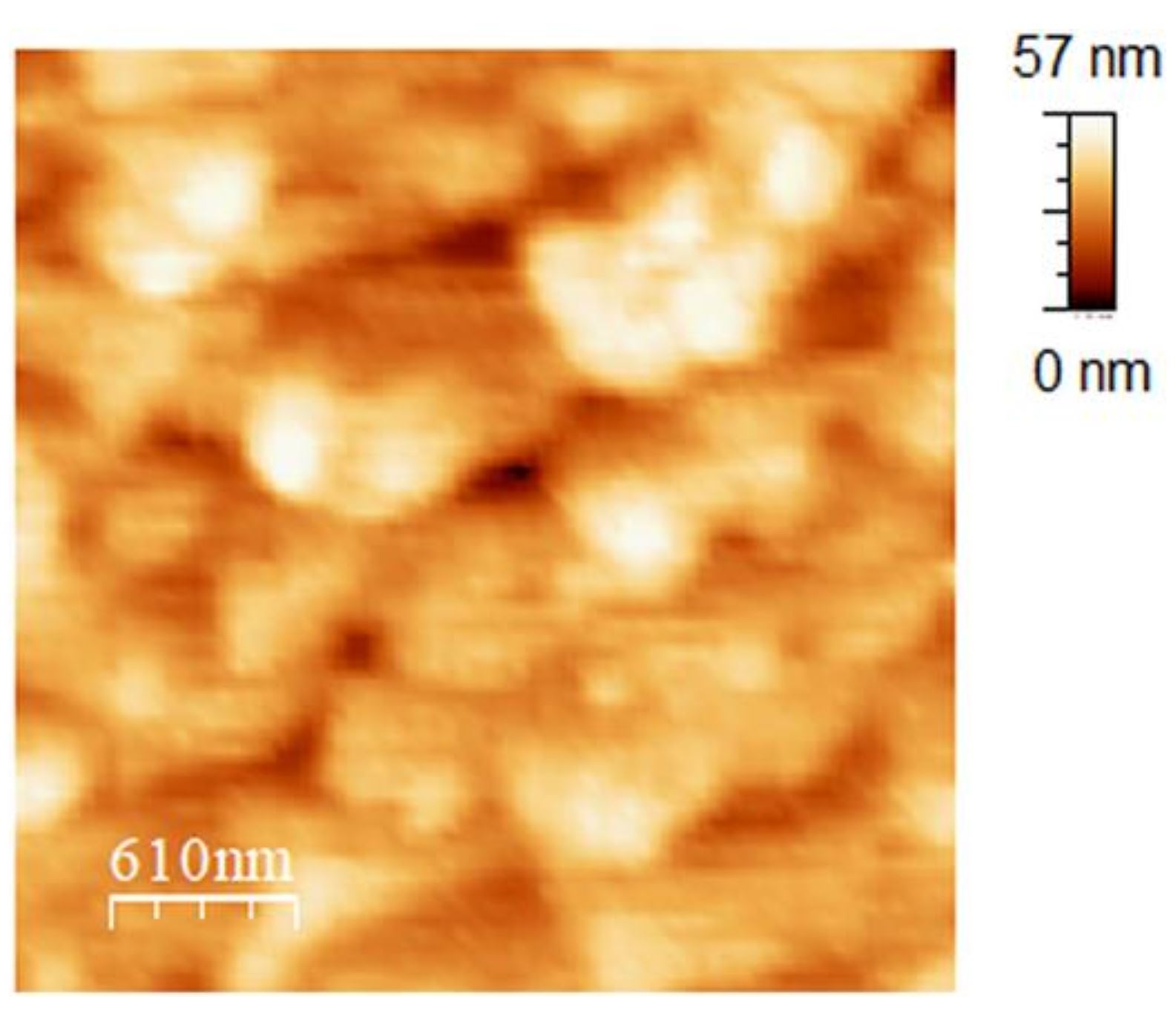
Figure 4 from Ryo Yanagisawa et al. (2024) “The interface between ice and alcohols analyzed by atomic force microscopy”:
AFM image (3.5 × 3.5 µm2) of the ice surface obtained ice in 1-butanol at 267.7 K.
*Ryo Yanagisawa, Tadashi Ueda, Kei-ichi Nakamoto, Zhengxi Lu, Hiroshi Onishi, Taketoshi Minato
The interface between ice and alcohols analyzed by atomic force microscopy
Journal of Chemical Physics 161, 024702 (2024)
DOI: https://doi.org/10.1063/5.0211501
The article “The interface between ice and alcohols analyzed by atomic force microscopy” by Ryo Yanagisawa, Tadashi Ueda, Kei-ichi Nakamoto, Zhengxi Lu, Hiroshi Onishi, Taketoshi Minato is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.